Therapy path classification in Parkinson’s disease: A clinical investigation using EMG and motion-based monitoring
With the global prevalence of Parkinson’s disease (PD) rising rapidly, there is a growing urgency to improve how symptoms are monitored and treatment decisions are made. This white paper presents a pioneering clinical investigation into the use of electromyography (EMG) and motion-based technology for therapy path classification in Parkinson’s care. The study examines how combining objective sensor data with patient-reported information can improve clinical evaluations and support more informed therapy decisions.
Purpose of this clinical investigation
PD is a chronic, progressive neurodegenerative disorder characterized by motor symptoms, such as tremor, rigidity, and bradykinesia [1]. The cornerstone of pharmacological treatment is dopaminergic therapy, with levodopa being the most effective agent [2]. Medication dosing is individualized and adjusted over time based on disease progression and symptom burden [3].
In advanced stages of PD, when oral or transdermal medications fail to provide adequate symptom control, device-aided therapies such as deep brain stimulation (DBS) and continuous dopaminergic infusion treatments may be considered.
Despite advancements in therapeutic options, the assessment of treatment efficacy remains predominantly subjective, largely depending on patient-reported symptoms and clinical assessments by healthcare professionals that capture only single time points. This approach may fail to capture the full spectrum of symptom fluctuations, potentially complicating the optimization of treatment plans, and may lead to inaccuracies in the evaluation of treatment outcomes.
It has previously been validated that Adamant Health’s EMG- and motion-based technology enables accurate and objective evaluation of motor symptoms in patients with PD [4].
This clinical investigation, the first of its kind, focuses on the benefits of the method in clinical care. It aims to examine whether EMG- and motion-based symptom analysis, combined with other information in the symptom report, can support clinical evaluation and therapy decisions in clinical care. The clinical evaluation and therapy decisions are closely linked to the classification of patients into different therapy paths based on their current symptom severity and fluctuation.
Projected growth in Parkinson’s disease prevalence by 2050
PD affects approximately 1% of individuals over 60, with prevalence rising due to aging populations [5] [6]. According to a recent study published in the British Medical Journal [7], global PD prevalence is expected to increase, with cases expected to double across all ages and sexes, reaching approximately 25.2 million by 2050.
The study indicates that PD will increase in all countries, with the greatest rise occurring in regions with middle socio-demographic indices and rapidly aging populations.
In Europe, Germany is projected to have the highest number of PD cases (≈574,000) by 2050, followed by France (≈356,000) and both Spain and the UK (≈307,000) [7].
Contributing factors include physical inactivity, poor diets and metabolic disorders, as well as emerging risks, such as environmental toxins and climate change. These trend upward with increasing industrialization and urbanization.
Traditional and technology-enhanced Parkinson’s evaluation
The traditional gold standard for assessing PD symptoms primarily relies on subjective evaluations during in-clinic visits. Clinical rating scales, such as the Unified Parkinson’s Disease Rating Scale (UPDRS), may be used to score the severity of symptoms and evaluate treatment efficacy. [8] [9] This approach, however, has clear limitations, often resulting in an incomplete assessment of disease progression.
Adamant Health’s unique data-analysis technology combines surface EMG and kinematic measurements to objectively quantify a PD patient’s neuromuscular and motor function. The digital health solution combines a wearable sensor (CE-marked medical device), which is attached to the patient’s arm for a long-term recording in clinical or non-clinical environments; a patient diary, which allows the patient to report medication intakes, symptoms and overall moods and feelings during the measurement period; and cloud-based analysis, providing a detailed symptom report.
Figure 1: Adamant Health’s digital health solution combining a wearable sensor, patient diary and cloud-based analysis to provide a detailed symptom report
The cloud-based analysis module (CE-marked medical device) analyzes the collected EMG and acceleration measurement data for usage in the symptom report. In addition, the symptom report also contains information from the patient diary, patient background information provided by the healthcare professional, as well as observations from the data analyst involved in the creation of the report. The report contains a visualization of the information and the interpretation of the results to be utilized by a clinician in patient care.
Unique clinical investigation of EMG- and motion-based PD monitoring
To date, no clinical study has investigated how EMG- and motion-based symptom analysis can support clinical evaluation and therapy decision-making in clinical care.
Healthcare systems are already strained by a shortage of specialists and the large number of Parkinson’s patients. Given the growing number of individuals expected to suffer from PD, healthcare professionals risk becoming even more overburdened than they already are today. Adding clinically meaningful data through the use of technology aims to ease clinicians’ workloads and enhance diagnostic efficiency.
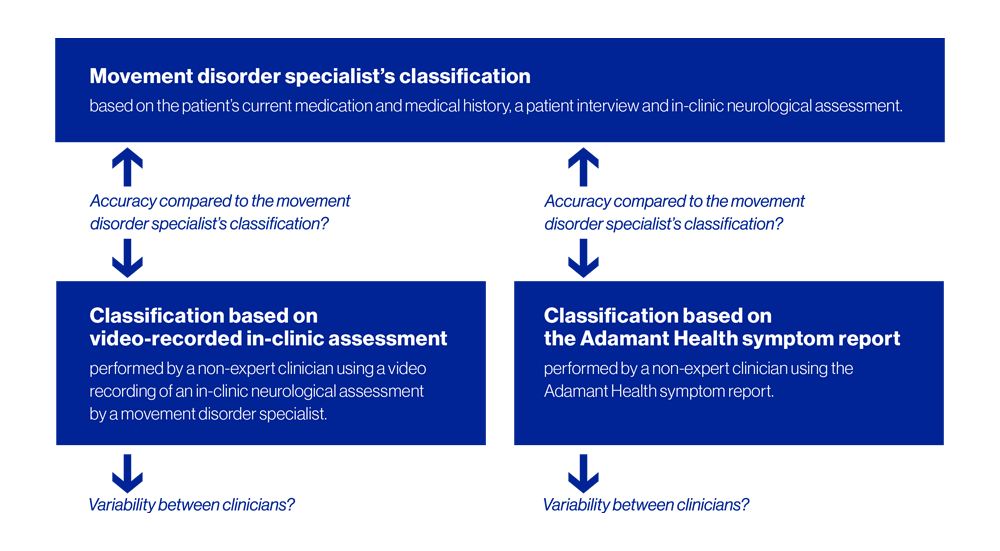
This investigation evaluates the value of Adamant Health’s symptom measurement and analysis in classifying patients into appropriate therapy paths compared to neurological examinations during traditional clinical visits.
Objectives and hypotheses of the clinical investigation
The primary investigation objective is to determine if the patient’s therapy classification is equally or more accurate using the muscle activity and movement data collected with a wearable EMG and motion sensor and analyzed by Adamant Health’s highly advanced mathematical algorithms compared to in-clinic neurological assessments.
The primary hypothesis is that classification accuracy using the wearable sensor and algorithm-based analysis is equally or more accurate compared to in-clinic neurological assessments. This will be tested using a two-sided 95% confidence interval (CI) for the difference in accuracy percentages between the methods. Non-inferiority will be concluded if the lower bound of the CI is equal to or greater than the pre-specified margin of -15%. The primary endpoint, classification accuracy, will be analyzed using the Newcombe method.
The study also investigates the variability in treatment path classification among non-expert clinicians and the impact of therapy changes on the patient’s quality of life.
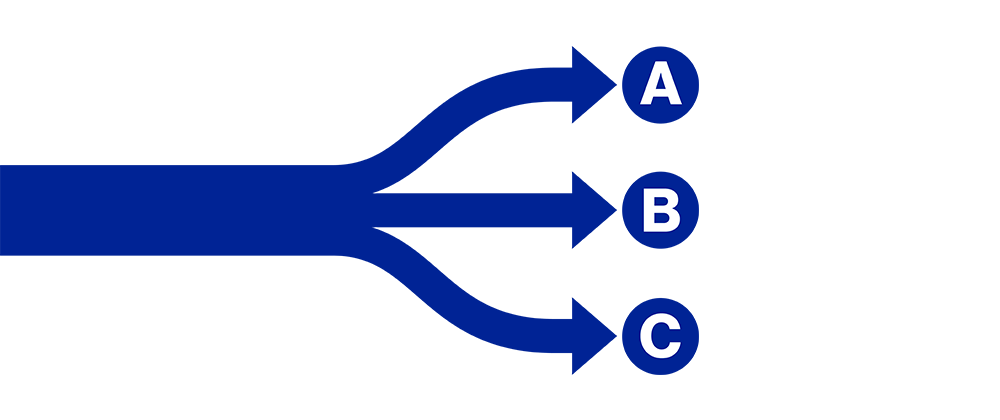
Figure 2: Primary and secondary objectives of the investigation
Primary outcome evaluation criteria
The primary outcome variable of the study is to evaluate whether the patient has been classified into the correct treatment path:
A: Motor symptoms are under control. The patient does not need therapy adjustment.
B: Therapy adjustment is needed for better motor symptom control. Possible motor fluctuations.
C: Oral medication is not sufficient any longer or is causing adverse side effects. The patient requires further evaluation for device-aided therapy options.
Secondary and exploratory objectives
The secondary endpoint objective relates to the variability in treatment path classification among clinicians, with the aim of determining if there is less variability in the classification of the therapy path when using Adamant Health’s symptom report or the in-clinic neurological assessment.
One of the exploratory endpoints of the investigation is to evaluate the effect of treatment adjustments on the patient’s symptoms and quality of life.
Additionally, the study collects clinician and patient feedback on the value of the symptom report in enhancing clinician-patient communication and information sharing.
References
[1] J. Jankovic, “Parkinson’s disease: clinical features and diagnosis,” J. Neurol. Neurosurg. Psychiatry 79(4), 368–376 (2008).
[2] L. Buyan-Dent, T. Mangin, and K. M. Shannon, “Pharmaceutical treatment of Parkinson’s Disease,” Pract. Neurol. 32–37 (2018).
[3] D. Grosset, K. Grosset, M. Okun, and H. Fernandez, Parkinson’s disease – Clinician’s desk reference (Manson Publishing Ltd., London, 2009).
[4] S. Rissanen, M. Koivu, P. Hartikainen, and E. Pekkonen, “Ambulatory surface electromyography with accelerometry for evaluating daily motor fluctuations in Parkinson’s disease,” Clin. Neurophysiol. 132, 469–479 (2021).
[5] L. De Lau and M. Breteler, “Epidemiology of Parkinson’s disease,” Lancet Neurol. 5, 525–535 (2006).
[6] E. Dorsey, F. Constantinescu, J. Thompson, K. Biglan, R. Holloway, K. Kierbutz, F. Marshall, B. Ravina, G. Schifitto, A. Siderowf, and C. Tanner, “Projected number of people with Parkinson’s disease in the most populous nations, 2005 through 2030,” Neurology 68, 384–386 (2007).
[7] D. Su, Y. Cui, C. He, P. Yin, R. Bai, J. Zhu et al., “Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: modelling study of Global Burden of Disease Study 2021,” BMJ 388, e080952 (2025).